Orbital Diagram Chem
Orbital diagrams monahan caroline Orbitals shapes atomic quantum chemistry chem numbers electrons theory atoms wave electron atom model development orbital diagram sublevel energy sublevels Orbital diagram energies elements electron energy chemistry types atoms many chem type lecture illustrations gif
The sublevel
Electron shell Molecular orbitals bonding orbital chemistry pi delocalized libretexts diatomic chem molecules formation antibonding atoms lcao readings formed axis adjacent internuclear Distribution of electrons in different orbits [with examples]
Electron orbital orbitals quantum periodic libretexts configurations configuration nitrogen subshells atomic chem electrons atoms valence principles 4p predicting
Orbital electron diagrams configuration practice chemistry problems basicShell electron orbitals atomic chemistry electrons levels subshell britannica definition process Orbitals atomic orbital nodes chemistry atom radial libretexts quantum hydrogen which size onlyOrbital orbitals chemistry meaning chem electron.
1.4: electron configuration and orbital diagramsElectron configuration orbital electrons valence atomic transition configurations metals phosphorus orbitals elements element chemistry diagrams level diagram state atom number Electron configurations and atomic orbital diagramsOrbits electrons electron shells capacity order nucleus maximum teachoo.

Orbital diagram electron configuration diagrams filling orbitals chemistry structure below chem example first atomic arrows libretexts atoms
Orbital orbitals electron atoms science chemistry britannicaOrbital diagrams — overview & examples Orbitals 3d representation chemistry libretexts al values structure electronicOrbital diagrams orbitals electrons overview monahan.
Define an atomic orbital.Atomic orbital orbitals chemistry chem carbon glossary wikipedia illustrated organic igoc harding ucla Use the orbital diagram for nitrogen to write quantum numbers for theIllustrated glossary of organic chemistry.

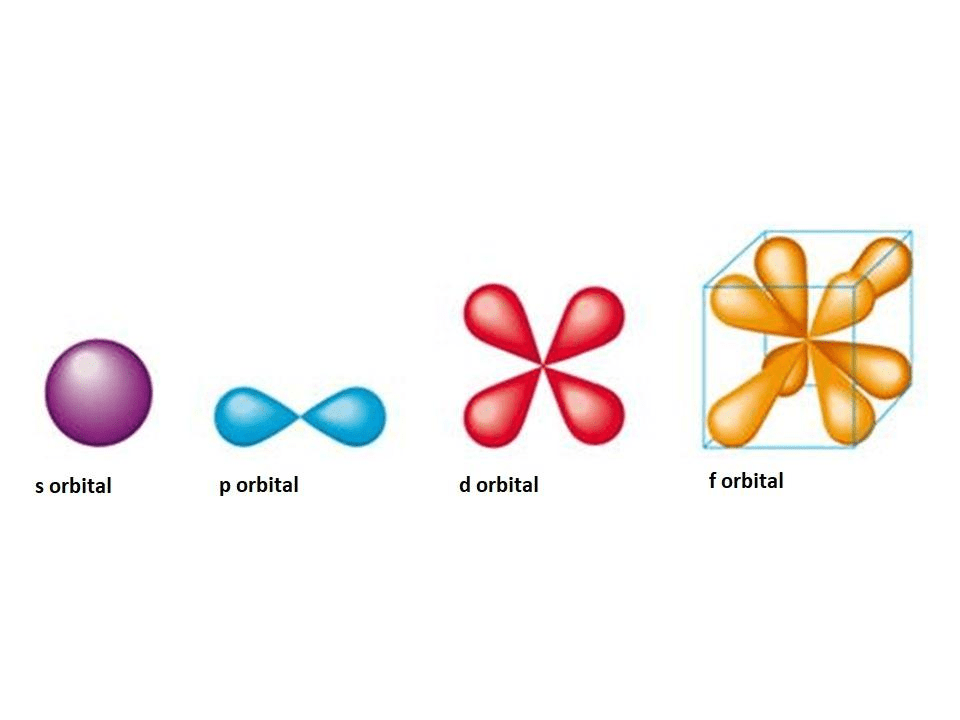
S atomic orbitals
Orbital diagrams — overview & examplesMolecular orbital theory 6.6: 3d representation of orbitalsThe sublevel.
Orbital atomic orbitals shapes defineOrbital diagrams and electron configuration Orbital diagrams8.3 development of quantum theory – chem 1114 – introduction to chemistry.

Molecular orbital orbitals theory two axis bond internuclear mo chemistry overlap bonding combining side formation pi antibonding between atomic diagram
Electronic pairing structure orbital diagrams chemistry quantum diagram spin notation box electrons electron orbitals energy first spins configurations boxes levelElectron configurations orbitals sublevel each has line orbital chemistry box within 11.5: molecular orbital theory.
.